A Short Course on “3D Bioprinting for Pharmaceutical and Biomedical Applications”
Descripción
About this course:
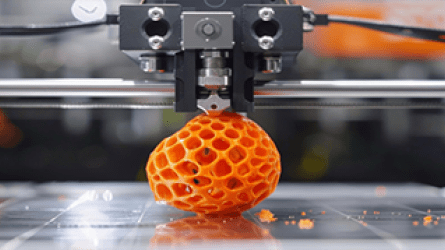
Three-dimensional (3D) bioprinting is an emerging technology with the potential to print tissues and organs, as well as pharmaceutical products. Thus, it is gaining significant interest in the field of human healthcare. This course aims to provide new knowledge and core technical skills in bioprinting suitable for pharmaceutical and biomedical applications. This short course covers the fundamentals and latest advancements in bioprinting technology and its translational applications. This two-day course is ideal for students, researchers, clinicians, and industrial personnel interested in learning about this cutting-edge bioprinting technology and receiving hands-on training from global leaders in academia and industry.
Objetivos
The objective of this course is to provide new knowledge and core technical skills in bioprinting that are suitable for pharmaceutical and biomedical applications.
Resultados del aprendizaje y tipo de logro
Learn about the working principles, technologies, advantages, and limitations of bioprinting and its applications in healthcare.
Learn to identify the appropriate technique and printer for your healthcare research.
Gain hands-on experience printing 3D constructs using bioinks.
Requisitos previos de acceso y criterios de admisión
This two-day course is ideal for students, researchers, clinicians, and industrial professionals interested in learning about cutting-edge bioprinting technology. Participants will receive hands-on training from global leaders in academia and industry. Participants should have prior knowledge of and experience in fields such as medicine, pharmacy, biotechnology, bioengineering, materials science or related subjects.
Required age: between 25 and 64 years old.
Nivel de la experiencia de aprendizaje según marco de cualificaciones EQF, European Qualifications Framework
EQF 7
MECU 7
MECES 3
Marcos competenciales ESCO, European Skills, Competences, Qualifications and Occupations
ESCO: pharmacotherapy
Knowledge and Skills on Biomedical Science
Pruebas evaluación
- Evaluaciones Escritas: 1.Exámenes de Opción Múltiple
Otras pruebas de evaluación
Exam will be conducted in EnglishPúblico objetivo al que está dirigida la actividad
- Alumnado universitario
- Profesorado
- Profesionales
Organiza
Colabora
Directoras/es
Murugan Ramalingam is a Ikerbasque Professor at the University of the Basque Country (UPV/EHU) (Spain). Group Leader at JRL Bioprinting and Advanced Pharma Development, a Joint Venture of TECHNALIA,. Concurrently, he is an affiliated faculty at Bioaraba Health Research Institute and at CIBER-BBN, Instituto de Salud Carlos III (Spain), an Adjunct Professor at Benzhou Medical University (China), and a Visiting Professor at Furtwangen University (Germany). He has also held an Adjunct Professorship at Tohoku University (Japan), Professeur des Universite at the Faculté de Chirurgie Dentaire, Université de Strasbourg (France), and High-End Foreign Expert at Chengdu University (China). Prior to this, he worked at the Dankook University (South Korea) as Professor, at the Université de Strasbourg (France) as Associate Professor, and at the WPI-AIMR (Japan) as Assistant Professor. He has also worked at the National Institute of Standards and Technology (NIST) and the National Institutes of Health (NIH), under the U.S. National Academies Associateship program. He is the author of ~380 publications, 15 textbooks and 50 book chapters with h-index of ~52/~18000 citations. Research Line: Tissue Engineering and Regenerative Medicine

José Luis Pedraz Muñoz
University of the Basque Country (UPV/EHU)
NOMBRE: José Luis APELLIDO: Pedraz Muñoz FECHA DE NACIMIENTO: 22-06-1959 NÚMERO DE TELÉFONO: +34 653593042 CÓDIGO POSTAL: 01240 CÓDIGO UNESCO: 3208-3209 3.1 TÍTULO DEL CARGO Catedratico de Farmacia y Tecnología Farmacéutica FORMACIÓN/ENTRENAMIENTO (Comienza con el bachillerato u otra formación profesional inicial, como enfermería, e incluye la formación postdoctoral). INSTITUCIÓN Y LUGAR AÑO DE TITULACIÓN CAMPO DE ESTUDIOS 1) Facultad de Farmacia Universidad de Salamanca: Licenciado en Farmacia. Farmacia de Junio de 1981 2) Ministerio de Educación: Especialista en Farmacia Hospitalaria. En 1990 Farmacia Hospitalaria. 3) Ministerio de Educación: Especialista en Farmacia Industrial y Galénica: En el año 2000. Farmacia Galénica. 4) Ministerio de Educación: Especialista en análisis y control de fármacos y medicamentos en el 2002. Análisis y Control de Medicamentos 5) Facultad de Farmacia Universidad de Salamanca: Doctorado en Farmacia en 1984. Cargos y honores. Enumere en orden cronológico los cargos anteriores, concluyendo con su cargo actual. Enumere los honores que haya recibido. Incluya la pertenencia actual a cualquier comité asesor público o privado. Profesor asociado Universidad de Salamanca 1/5/86-1/9/90 Profesor asociado Univ
Ponentes

Sandra Ruiz Alonso
I am a biotechnologist with a PhD in Tissue Engineering, specialized in 3D bioprinting and regenerative medicine. My background includes experience in drug discovery, biomaterials development and organ-on-a-chip technologies, which has given me a multidisciplinary perspective on biomedical research. I currently work as a postdoctoral researcher at the NanoBioCel group, where I focus on the design of advanced bioinks and the development of 3D bioprinted skin models as alternatives to animal testing. My work combines the formulation of hydrogels, 3D printing strategies and cell culture techniques to create functional tissue models with applications in drug testing and regenerative medicine. I am also involved in collaborative projects that integrate bioprinting with microfluidic platforms to develop more physiologically relevant models.

Nicola Paccione Basmadji
Nicola Paccione is a researcher at the Pharma Lab Services area at Tecnalia Research and Innovation and at the University of the Basque Country, his current work specializes in the development of 3D-printed drug formulations for personalized therapies. With a background in pharmaceutical innovation, he previously conducted research at the Complutense University of Madrid, focusing on nanotechnology approaches for the treatment of neurodegenerative diseases. Nicola brings expertise at the intersection of advanced manufacturing and pharmaceutical science, contributing to the design of novel therapies and drug delivery systems.

Deepak Kalaskar
Dr. Deepak Kalaskar is a Professor of Bioengineering at University College London (UCL) with over 25 years of multidisciplinary research experience. He began his career as a Research and Development Scientist at BASF Coatings Pvt Ltd, a leading German multinational company. Driven by his fascination with biological coatings, Dr. Kalaskar pursued a PhD in Biomedical Materials at the University of Manchester, UK. After completing his PhD, he held various academic positions in the UK and Belgium, contributing to numerous research projects. These include high-throughput culture techniques for embryonic stem cells as part of the Northwest Stem Cell Consortium in the UK, the development of nanomaterial coatings for orthopedic implants at the Nano Science and Condensed Matter Lab in Belgium, and the application of 3D printing technologies for cell injection and osteochondral plugs for knee repair at the ARUK Tissue Engineering Centre in Newcastle. Dr. Kalaskar's research focuses on developing novel biomaterials and their applications, optimizing 3D bioprinting processes and bio-inks, and designing and manufacturing innovative medical devices and implants using cutting-edge 3D technologies such as imaging, scanning, and printing.

Sean Murphy
Dr. Sean Murphy is a Professor of Regenerative Medicine, at the Wake Forest Institute for Regenerative Medicine (WFIRM). He received his B.Sc. in Molecular Biology from the University of Western Australia in 2006 and his Ph.D. in Stem Cell Research from Monash University in 2011. Dr. Murphy’s research focus is the field of lung regenerative medicine; leading a team developing regenerative medicine and tissue engineering strategies for treating and preventing lung disease. Dr. Murphy specific research interests include; developing anti-inflammatory stem cell therapies, bioengineered in vitro models for lung disease modeling and drug discovery, 3D bioprinting of functional airway tissue, and functional biomaterial development.
Murugan Ramalingam is a Ikerbasque Professor at the University of the Basque Country (UPV/EHU) (Spain). Group Leader at JRL Bioprinting and Advanced Pharma Development, a Joint Venture of TECHNALIA,. Concurrently, he is an affiliated faculty at Bioaraba Health Research Institute and at CIBER-BBN, Instituto de Salud Carlos III (Spain), an Adjunct Professor at Benzhou Medical University (China), and a Visiting Professor at Furtwangen University (Germany). He has also held an Adjunct Professorship at Tohoku University (Japan), Professeur des Universite at the Faculté de Chirurgie Dentaire, Université de Strasbourg (France), and High-End Foreign Expert at Chengdu University (China). Prior to this, he worked at the Dankook University (South Korea) as Professor, at the Université de Strasbourg (France) as Associate Professor, and at the WPI-AIMR (Japan) as Assistant Professor. He has also worked at the National Institute of Standards and Technology (NIST) and the National Institutes of Health (NIH), under the U.S. National Academies Associateship program. He is the author of ~380 publications, 15 textbooks and 50 book chapters with h-index of ~52/~18000 citations. Research Line: Tissue Engineering and Regenerative Medicine

Denis Scaini
Dr. Denis Scaini holds a Degree in Materials Engineering and a PhD in Nanotechnology. In 2010, he shifted his focus to neuroscience research, working in a multidisciplinary environment that includes neurophysiology, cell biology, nanotechnology, and materials science. His research primarily uses advanced biofabrication and nanotech tools to investigate unexplored aspects of neuronal cell physiology in both 2D and 3D cellular models. His goal is to uncover the key mechanisms governing cellular interactions and functions, with the aim of establishing new methods to promote tissue development and modulate functionality. As an Ikerbasque Researcher, he leads the neuroregenerative unit at the Joint Research Laboratory for Advanced Pharma Development (JRL-APD) at the University of the Basque Country, Spain.

Andrew Shevchuk
Dr. Andrew Shevchuk – Associate Professor, Department of Metabolism, Digestion and Reproduction, Faculty of Medicine, Imperial College London. Graduated in specialised computer systems at Kyiv Polytechnic Institute, Ukraine in year 2000, I came for a PhD to Imperial College London where I started to develop prototypes of a Scanning Ion Conductance Microscope (SICM) and wrote the microscope control software. Shortly after, my early SICMs were installed in our collaborators’ labs at the University of Kanazawa, Japan and Academia Sinica, Taiwan, and I co-founded the first spin-out company to produce SICM equipment, but remained in academia. As a post-doctoral researcher, I improved the topographical resolution of SICM by two orders of magnitude, speed up the imaging and combined SICM with fluorescence imaging to enable Correlative Live Imaging. In 2012, I went to the Institute for Life Sciences, University of Southampton, to start my own lab and was head-hunted back to Imperial College a few years later. Since then, I continued working on expanding SICM toolbox with new combinations such as Electrochemical Microscopy, developed new multifunctional probes for multiparametric imaging, performed research in cell membrane dynamics .

Thomas Webster
Thomas J. Webster’s (H index: 134) degrees are in chemical engineering from the University of Pittsburgh (B.S., 1995; USA) and in biomedical engineering from RPI (Ph.D., 2000; USA). He has formed over a dozen companies who have numerous FDA approved medical products currently improving human health in over 30,000 patients. He is currently helping those companies and serves as a professor at Brown University, Saveetha University, Hebei University of Technology, UFPI, and others. Dr. Webster has numerous awards including: 2020, World Top 2% Scientist by Citations (PLOS); 2020, SCOPUS Highly Cited Research (Top 1% Materials Science and Mixed Fields); 2021, Clarivate Top 0.1% Most Influential Researchers (Pharmacology and Toxicology); 2022, Best Materials Science Scientist by Citations (Research.com); and is a fellow of over 8 societies. Prof. Webster is a former President of the U.S. Society for Biomaterials and has over 1,350 publications to his credit with over 55,000 citations. He was recently nominated for the Nobel Prize in Chemistry. Prof. Webster also recently formed a fund to support Nigerian student research opportunities in the U.S.

Jon Zarate Sesma
UPV/EHU
Jon Zarate Sesma (Lekeitio, 1979) es miembro del Departamento de Farmacia y Ciencias de los Alimentos de la UPV/EHU. Es profesor de la Facultad de Farmacia y vicerrector del área de Euskera y Formación Continua (2014-2021). Doctor en Farmacia (UPV/EHU, 2007) y director de la mejor tesis sanitaria en euskera, Koldo Mitxelena 2016. En los últimos años ha estado investigando en el desarrollo de transportadores no virales para terapia génica. Tiene publicados más de 20 artículos en revistas internacionales de investigación de alto nivel y una producción científica en euskera similar a la internacional en revistas como Ekaia o Elhuyar.

Yu Shrike Zhang
Dr. Zhang is currently Associate Professor in the Department of Medicine at Harvard Medical School and Associate Bioengineer in the Division of Engineering in Medicine at the Brigham and Women’s Hospital. Dr. Zhang is directing the Laboratory of Engineered Living Systems (www.shrikezhang.com), where the research is focused on innovating medical engineering technologies, including 3D bioprinting, organs-on-chips, microfluidics, and bioanalysis, to recreate functional tissues and their biomimetic models, for applications in regenerative medicine and personalized medicine. He is an author of >350 peer-reviewed publications citations ~47,000, h-index=107). His scientific contributions have been recognized by >50 international, national, and regional awards
Precios matrícula
No cabrá devolución de la matrícula en el caso de haberse iniciado la impartición de la microcredencial.
| Matrícula | Hasta 21-10-2025 |
|---|---|
| 84,96 EUR |
Lugar
Centro de Investigación Lascaray Ikergunea
Avenida Miguel de Unamuno 3 01006 Vitoria-Gasteiz
Araba
Centro de Investigación Lascaray Ikergunea
Avenida Miguel de Unamuno 3 01006 Vitoria-Gasteiz
Araba











